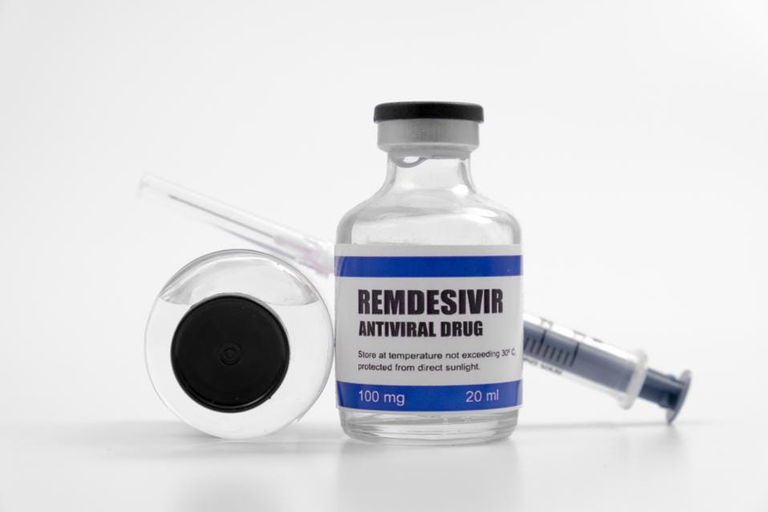
The experimental antiviral medication remdesivir, also known by the trade name Veklury, was approved by the US Food and Drug Administration (FDA) for treatment against COVID-19 in an emergency in May 2020.
In October 2020, a few months later, it had already gotten final clearance.
Despite data demonstrating it lacks effectiveness and can lead to high rates of organ failure, it is still the predominant therapy for COVID-19 in hospitals today. However, some people, like John Beaudoin, are using Twitter to demand that the medication be the subject of a criminal probe, citing information that may have killed 100,000 people in the US. It raises questions about whether American health officials have exclusively concentrated on this and other medications that are just as hazardous.
The US District Court received a lawsuit from Beaudoin, believing that Gilead Sciences' remdesivir is to blame for a rise in acute renal failure (ARF) mortality in Massachusetts.
All death certificates for Massachusetts from 2015 through 2022 were obtained by Beaudoin through a Freedom of Information Act (FOIA) request. Nevertheless, it discovered that between January 1 and November 30, 2022, there were 1,840 more fatalities due to acute renal failure. In every age group over 15, it also showed an increase in acute rent insufficiency fatalities from 2015 to 2022, according to Beaudoin. At least 7,491 adverse medication reactions, including 560 fatalities, 550 significant cardiac conditions, and 475 acute renal injuries, have been reported to the World Health Organization (WHO) VigiAccess since that time through October 2021.
Just to give you an idea of the scope, from 1992 to October 13, 2021, only 5,674 adverse medication reactions with ivermectin were documented.
Ivermectin has received a lot of negative press throughout the epidemic despite having a high safety and effectiveness profile. Then there is the issue of price: Remdesivir has a price range of $2,340 to $3,120, whereas ivermectin has a treatment cost average of $58.
A research published in The Lancet reported no benefit clinical from the use of remdesivir in hospitalized patients, despite WHO updating its recommendations to encourage its use in mild or moderate COVID-19 patients who are at high risk of hospitalization in April 2022.
In addition, three deaths that occurred during the trial, according to the researchers, were due to remdesivir. There are several litigation active now. As is well known, the pharmaceutical industry has a significant impact on American politics. Remdesivir should not be used in patients with COVID-19, according to a WHO recommendation published in November 2020.
There is presently no proof that remdesivir increases patient survival and other outcomes. Might Gilead's deep political connections have affected government endorsements and suggestions?
A few questions arise. All that has to be said is that Donald Rumsfeld led Gilead from 1997 until 2001, when he joined the Bush administration.
Rumsfeld previously held the position of defense secretary under Presidents Gerald Ford (1975–1977) and George W. Bush (2001–2006). The narrative also has another unsettling element that includes helpless beings like children, who, as contrast to adults, do not control how much vaccination is given. Remdesivir was in fact given FDA approval in late April 2022 as the first and only COVID-19 medication for children under the age of 12, including newborns as young as 28 days.
If you consider that COVID-19 is seldom serious in children while remdesivir is ineffectual and runs the danger of significant and fatal side effects, this decision is all the more startling. What's worse is that youngsters can potentially utilize the medication outside of a hospital setting. If 7 out of 1,000 fatalities are related to drugs, as European researchers believed it's probable that more kids are harmed or killed as a result than are saved.
The FDA ought to have waited longer to assess the effects of early outpatient therapy on later ages. Hardly nothing has been written on kids and Remdesivir.
The approval was based on a 53 infant open-label, single-arm trial, of which 3 newborns died (6% of these infants perished), 72% had an adverse event, and 21% experienced a significant adverse event, according to Gilead's news statement. In conclusion, even if the Covid-19 Epidemic is stopped, we must continue to discuss the numerous contraindications brought on by the therapies tried to halt it, which have irreparably harmed millions of lives.
Congratulations @wolfplayzor! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 500 comments.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts:
Support the HiveBuzz project. Vote for our proposal!