Many of the reactions that occur in the body would happen at remarkably low rates under physiological conditions.
In chemistry, a catalyst is used to increase the rate of a given reaction. This is exactly how it works within the body. Enzymes are biological catalysts, used to speed up reactions that occur in the body to the necessary rates. They have numerous functions within the body, which are listed below:
- Increase reaction rates
- Increase reaction specificity
- Capacity for regulation
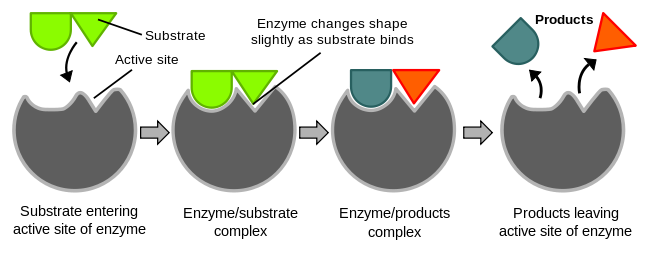
figure 1. Enzyme catalysis
Which chemical reactions will occur?
For a reaction to proceed it must be energetically favourable. Energy is considered in two main forms:
- Enthalpy (H) is heat and a change in enthalpy (ΔH) occurs when a reaction gives off, or takes in heat. Release of heat (ΔH is negative) is favourable.
- Entorpy (S) is disorder and a change in entropy (ΔS) occurs when a reaction leads to increased or decreased order. Increase in disorder (ΔS is positive) is favourable.
The balance between entropy and enthalpy changes determines whether a reaction can proceed. Chemical reactions will proceed if there is a reduction in the energy state of system either through loss of enthalpy (-ΔH) or gain of entropy (+ΔS). Enthalpy and entropy are related through the Gibbs free energy (G) equation.
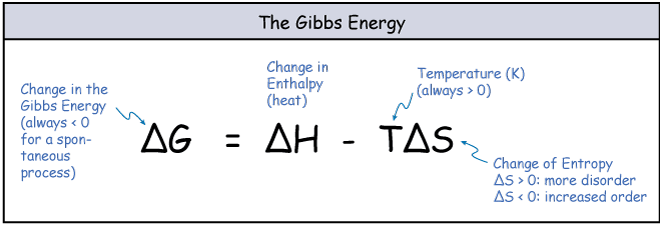
figure 2. Gibbs Free Energy equation
If the Gibbs free energy for a reaction is negative then it is thermodynamically favourable. Enzymes cannot drive thermodynamically unfavourable reactions, so for any enzyme catalysed reaction ΔG < 0.
Do all thermodynamically favourable reactions proceed?
Not necessarily. Many reactions with a negative ΔG will not occur due to a kinetic barrier, the activation energy. The transition state is the highest energy intermediate in the reaction. An input of energy is needed to distort the substrate of the reaction to form the transition state, and this is called the activation energy. If the activation energy is very large a reaction will not occur, it is kinetically unfavourable. This is shown in figure 2.
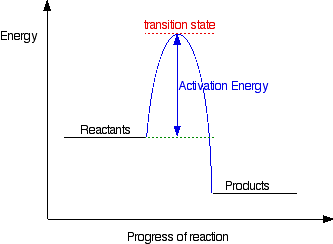
figure 3. A reaction pathway
How do catalysts work?
A catalyst reduces the energy of the transition state of a reaction. This in turn decreases the required activation energy, thus overcoming the kinetic barrier of a reaction. However, a catalyst does not change the ΔG of a reaction or alter its equilibrium position. This is depicted in figure 3 below.
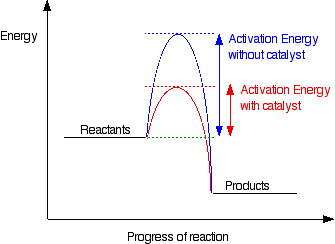
figure 4. Activation energy uncatalysed vs catalysed
How do enzymes act as catalysts?
An enzyme will bind to the substrate, transition state and products. By stabilising the transition state, an enzyme reduces the activation energy, allowing reactions to proceed at faster rates.
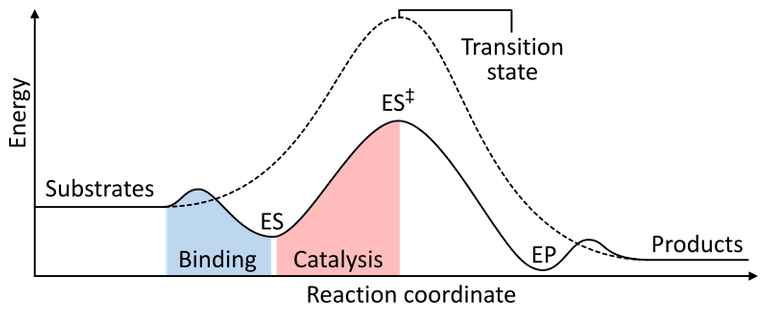
figure 5. Enzyme catalysis reaction pathway
Figure 5 shows the uncatalysed reaction with a dotted line, and the catalysed reaction with the solid line. ES is when the enzyme binds to the substrates, with ES++ being the stabilised transition state. The catalysed transition state is at a much lower energy than uncatalysed, thus the activation energy has been reduced. EP is the enzyme and product complex.
The active site is the region of the protein where the reaction takes place. It must:
- Bind to the substrate selectively = be complementary to the substrate.
- Stabilise the transition state. This could involve distortion of bonds in the substrate, donation and removal of protons or placing substrates in the correct orientation.
- Release the product rapidly = not bind the product too tightly.
Models for enzyme reactions
There are two main models used to explain enzyme catalysis. These are shown in figure 6.
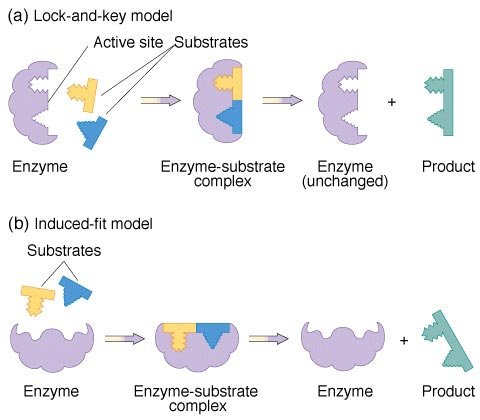
figure 6. Enzyme reaction models
The first model is known as the lock-and-key. It states that the enzyme active site is exactly complementary in shape to the substrates. Therefore, the substrates fit neatly together inside the active site, where the reaction proceeds until the product is released.
The second model is called the induced-fit. The main difference from the lock-and-key model is that the enzyme and substrates are not exactly complementary. As the substrate reaches the enzyme active site, this induces a change in the active site of the enzyme to allow the substrates to fit within it (as shown in figure 6). Once the product is released, the enzyme returns to its original conformation.
Neither of these models fully describes the mechanism of action of every enzyme. It appears that some enzymes operate in a manner very close to the lock-and-key model, while others are more suited to the induced-fit. Nonetheless, from both of these models it is clear that the shape of the active site is a critical factor in enzyme catalysis and speeding up biological reactions.
Why should we care?
There are huge numbers of enzymes within the human body, each of which is required for life to occur (reactions just wouldn’t occur fasts enough to sustain life otherwise). Each of these enzymes has specific mechanisms to reduce the transition state energy level (which are far too detailed for this post).
Many enzymes are subject to control (such as phosphorylation or allosteric regulation) within the body. By controlling enzymes, the body is able to regulate which reactions occur and which do not. While being a useful tool for the body, this also makes enzymes excellent drug targets, and understanding how enzymes work is crucial to being able to make effective drugs that disrupt enzymatic processes.
References:
Catalysis
Enzymes
Enzyme reaction models
Thank you for providing such informative stuff of biology.
Enzymes are really the Expressways of our body. They help a reaction to occur in our body. They can assist both Catabolic or Anabolic Reactions. Which in turn form the foundation stone of survival for a cell.
P.S. The article is a great Read and the Topic in itself is a fascinating one. Good Job @ovij :)
Thanks for the information. Glad you enjoyed the post, hopefully more to come!
Congratulations @ovij, this post is the sixth most rewarded post (based on pending payouts) in the last 12 hours written by a User account holder (accounts that hold between 0.1 and 1.0 Mega Vests). The total number of posts by User account holders during this period was 1464 and the total pending payments to posts in this category was $2609.25. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
You have a lot of awesome posts, but this is by far one of your best ones man. I needed this one today! Thanks.
This post recieved an upvote from minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond
I always appreciate your conclusion of "Why should we care". There is always something in there that I was unaware of. Thanks!
No problem, I try to make my posts are relatable as possible. Glad you enjoyed it!
This deserves some attention. Upvoted and resteemed :]
Thank you, much appreciated.