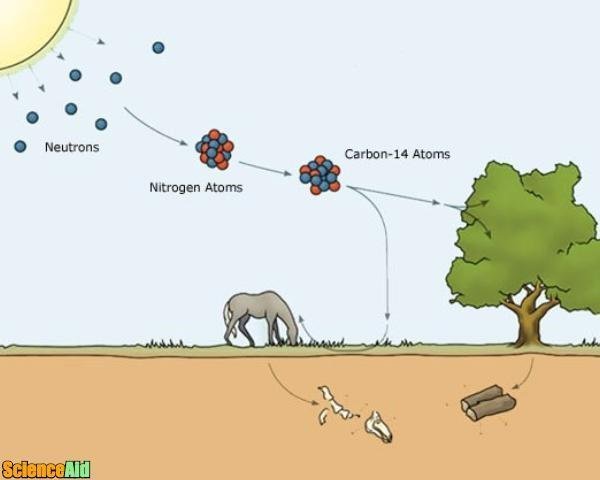
Scientists can use radiocarbon dating to help determine the age of organisms that once had life. This method can help date mostly anything from bone and cloth to wood and straw. Scientists look for levels of carbon-14, a rare form of carbon that begins to decay and disappear as soon as an organism dies. The older the object, the less carbon-14 it contains.
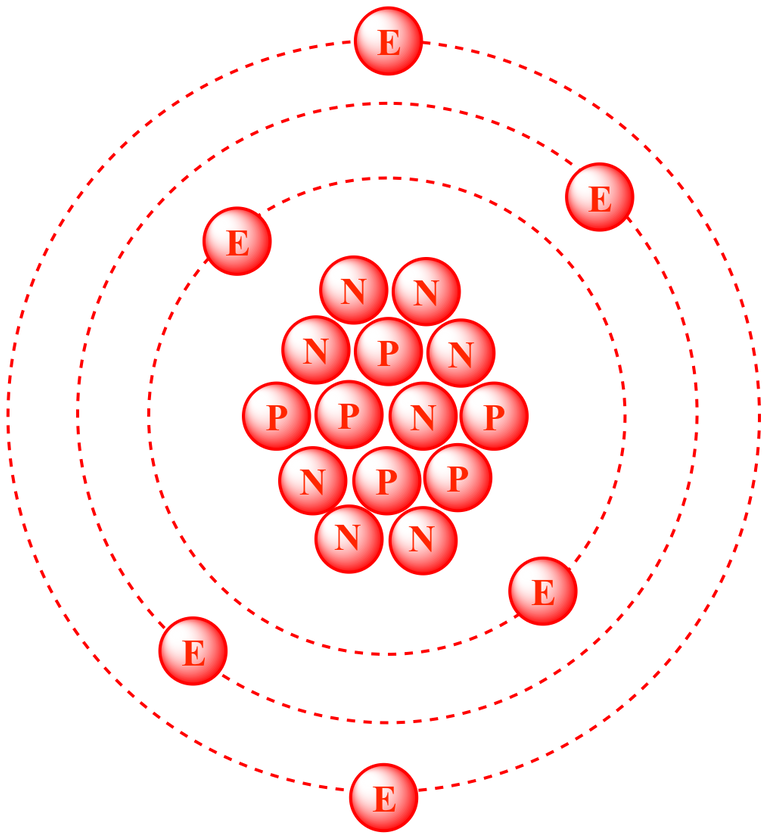
All living organisms on Earth are based on carbon. Normal carbon atoms (carbon-12) have 6 protons and 6 neurons. Yet, sometimes cosmic rays entering the Earth's atmosphere will bombard nitrogen atoms, charging them into a special type of radioactive carbon called carbon-14. Carbon-14 contains 2 more neutrons than normal carbon. Over the course of 5,730 years, half of any given sample of carbon-14 will decay back into nitrogen. In other words, carbon-14 is capable of a half-life of 5,730 years.
Animals and plants aren't capable of distinguishing between carbon-12 and carbon-14. Therefore, they consume both until they die. However, when a plant or animal dies, the levels of carbon-14 in the organism start to decline. (Carbon-14 begins to decay and, because the organism is no longer consuming carbon, it is not being replenished.)
During this same time, the amount of carbon-12 in the sample remains constant, as it is a stable molecule that doesn't decay. To determine the particular age of a dead object, scientists compare its ratio of carbon-14 to carbon-12 to the ratio in an equivalent living object.
If the dead object contains half the ratio of the living object, then it is approximately 5,730 years old, because carbon-14 has a half-life of 5,730 years. If the ratio is one-quarter the living comparison, then the object is precisely 11,460 years old. If the ratio is one-eighth, then the object is 17,190 years old. If an object is much older than 60,000 years, there isn't enough carbon-14 left to be used scientifically.
Radioactive uranium has a half-life of 704,000,000 years. It is often used to help date objects on a geologic scale.
Potassium-40, which is naturally found in the human body, contains a half-life of 1,300,000,000 years. It can and has been used to help date the beginning of life on the planet.
William F. Libby is credited as having developed radiocarbon dating in the 1940s-1950s at the University of Chicago. He was later awarded the Nobel Prize in Chemistry.
Since nuclear activity has changed what has been considered constant levels of radioactive material on Earth, anything that has died since 1940 will be more difficult to test with carbon dating.
Sources and further readings
American Chemical Society- Radiocarbon dating
University of Arizona
University of California Berkeley
Tufts University
Boston University
Wikipedia- Radioactive dating

Congratulations! This post has been upvoted from the communal account, @minnowsupport, by SunnyEgo from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
thanks, it's very interesting, although I knew about this method, I didn't know about Nitrogen becomes Carbon 14 because of cosmic rays, that's a new info for me. I really like how you explained everything keep do it with other science topics.
Wow, now this is something I never bothered to consider (probably because I've never been a natural science buff.) As someone who studied poetry in college and has an M.A. in Psych, I very much appreciated this little lesson! Thanks!