Hello, today I bring you a new chapter of my spectroscopy series. If you want to know more about this series, do not forget to read my previous articles.
One of the most famous techniques within the spectroscopy is the infrared, commonly called and better known as IR spectroscopy. This spectroscopic technique is based on absorption, that is, it studies the molecular behavior of a material that absorbs the infrared radiation of the spectrum. electromagnetic. And like most of the techniques I have described in previous articles, this is responsible for studying the chemical composition of a material and then explain what this technique is about.
The IR is used mainly by scientists dedicated to the area of chemistry, a primordial tool for them in the analysis of elements and any substance in different states of matter in both organic and inorganic chemistry. The technique collects specific datis of the vibrations of the atoms within the material molecule and, from this analysis, determines functional groups of different compounds. The analysis of the infrared light that interacts with this molecule is the basic principle of IR spectroscopy, and this can be studied in three ways when measuring, absorption, emission and reflection. Through this it is possible to observe structures of any chemical compound bound with covalent bonds, in this case the organic compounds.

IR spectrometer Licensed CC BY-SA 4.0
This spectrometer is undoubtedly a fantastic and extremely useful tool to be able to verify this type of analysis and obtain reliable results with a negligible margin of error.
We know that different chemical bonds have the capacity to absorb infrared frequencies and this technique shows the vibrations of the atoms of said frequency, which can be shown through "wave numbers" according to the type of link that contains the compound. It is also widely used for measuring the degree of polymerization in different polymers, this presents changes in the character of a certain bond and from this they are analyzed by measuring the specific frequency with time.
Great true!
IR spectroscopy has great advantages and is that we can use it to study any type of material in virtually all states of matter such as solids, liquids, solutions, films, pastes, fibers and gases that allow us to study through a selection of the technique sweeping
With the passing of the year the technique has been perfected enormously and has been used in various scientific fields, since at present various instruments can collect IR measurements at 32 times per second, while they can also make simultaneous measurements using other techniques, transforming the reactions of chemical compounds much faster and more accurately.
When molecules absorb frequencies of their structure, they tend to oscillate more broadly at specific frequencies, this means that when IR spectroscopy explodes in the molecules, the frequency of radiation absorbed by these molecules is at the frequency of vibration. the atoms, therefore, the energy within the molecules is affected the masses of the atoms and the vibratory coupling associated in this phenomenon.
A very important point to note is that the resonance frequencies are also strictly related to the strength of the link and the mass of the atoms at each end of it, then the frequency of the vibrations is associated with a particular normal mode of motion and a particular type of link.
We know that the technique determines the number of vibrational modes within the structure of a compound. In a specimen an "active IR" can be obtained, this means that the vibration mode is associated with the dipole change, usually a permanent dipole is not needed, since only a change in the moment of the dipole is required. Within the vibrational modes there are two types that are flexion and tension. The first one is originated particularly by the changes that occur in the angle where the bonds of each atom are formed, and the second one presents the change of interatomic distance between the bonds of two atoms.






CHn vibrations Licensed CC BY-SA 4.0
This translates into the following: when the infrared light hits a certain molecule, the bonds of the molecule absorb all the necessary energy of the infrared to transmit the signal and these respond vibrating and this is where scientists call these vibration modes as shows in the CHn figure.
The atoms in a CH 2 X 2 group, which are commonly found in organic compounds and where X can represent any other atom, can vibrate in nine different ways. Six of these vibrations involve only the CH 2 part: symmetric and antisymmetric of stretching, scissoring, rolling, moving and rotating, as shown below. Structures that do not have the two additional X groups connected have fewer modes because some modes are defined by specific relationships with those other attached groups. For example, in water, the modes of swing, movement and spin do not exist because these types of movements of the H represent a simple rotation of the entire molecule instead of vibrations within it.
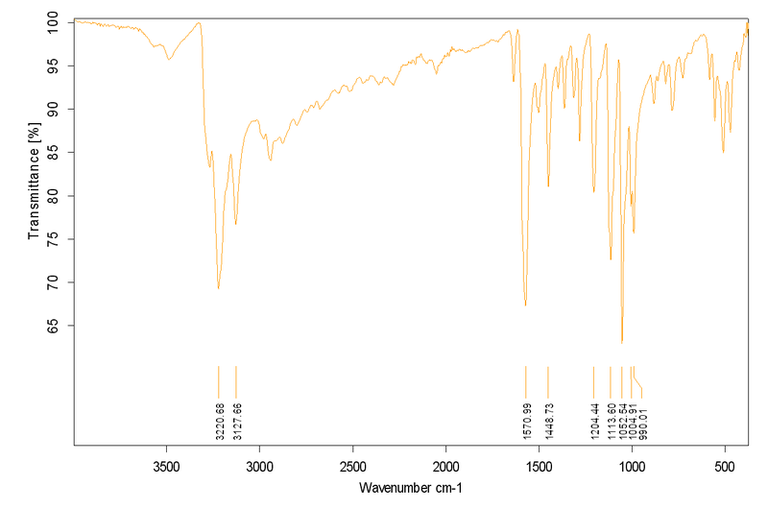
Este es un ejemplo del Espectro IR de cloruro de cobalto (III) de cis-diclorobis (etilendiamina) Licensed CC BY-SA 3.0
Now, after having explained the theory involved infrared spectroscopy comes the essential part and is what we all finally want to obtain by means of this technique, and we refer to the characteristic IR spectrum of a material being the fingerprint of said material, Each molecule has a characteristic IR spectrum, because all the molecules within the material present vibrations, which when activated generate the absorption of a certain wavelength of the electromagnetic spectrum corresponding to the infrared zone.
It should be noted that by performing the corresponding analysis of each wavelength that is absorbed in the infrared zone, we can obtain important information related to each molecule that makes up a substance or material.
The absorption bands is another important point to highlight in the IR theory, these bands are used to identify all types of molecular structures since the functional groups tend to generate characteristic bands of each material, interpreted in terms of intensity and frequency.
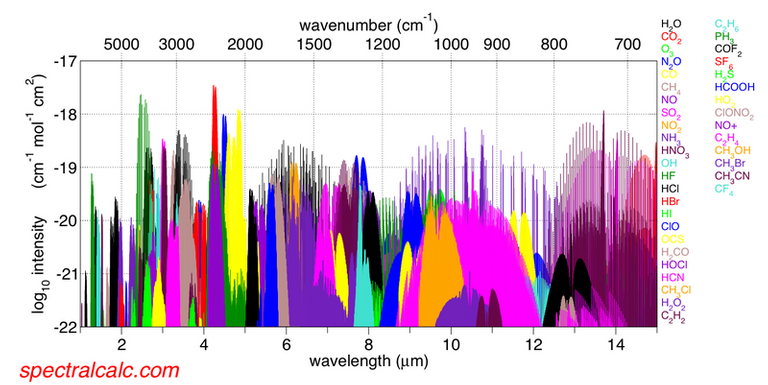
Infrared absorption bands Licensed CC BY-SA 3.0
Preparation of sample
For all techniques, a preliminary preparation of the sample of the material is necessary before proceeding to its analysis, in this case the IR spectroscopy is divided into 3 parts, liquid samples, solid samples and gaseous samples. Each one requires its respective characteristic preparation.
The preparation of the samples for this type of analysis is simple, we only have to take into account some important points, for example, to make the plates you must use materials like KBr, NaCl, since the salts are transparent to the radiation of the infrared
Two highly purified alkali halide plates, ie, salts such as sodium chloride NaCl and likewise potassium bromide KBr can be used in the liquid samples. Organic solvents such as chloroform should be used, because solvents containing water can dissolve the salts. The thickness should be in the range of 0.01-0.05 nm.

Typical IR solution cell. The windows are CaF2. Licensed CC BY-SA 4.0 Wiki
In solid samples, most researchers resort to crushing the sample completely with a substance called alkaline halide such as KBr. But also other people usually use another similar method, in this case they use a mineral oil and a very thin layer of a film from the mull is created and applied to the salt plates and measured. There are other methods since the IR technique is usually used mostly for solid materials for its effectiveness, other methods of preparation are: Dissolve the sample in a solvent that is completely free of water so that there is no chemical contact with the solvent and that the solvent does not damage the range you want to study, for example, this is usually placed a small drop of water. Solution in a metal plate made with salt and then the solvent tends to evaporate resulting in a thin film of solute.
Another method that is used when we have an amorphous solid is to deposit the sample in KBR or NaCl through the evaporation of the solution of the solid and must take into account that the film should not be very thick.
And to prepare a very used preparation method that receives the name of pressed pellet that consists in depositing a small amount of the specimen totally crushed or made dust, then it must be mixed with KBr and later it is compressed in a very thin and transparent pellet by means of of a press.
How to prepare a pallet in solid samples
The gaseous samples are quite simple to prepare, they do not require much work and it is basically the same preparation of a liquid sample, unlike a sample with a long distance of approximately 5-10 cm is needed, this is done since generally the gases have a very weak absorbance.
Infrared spectroscopy It is a highly versatile technique and is used in various fields of science because it has several important resources to identify materials, in addition to its easy handling is a very reliable technique, fast for any type of measurement and provides undoubted quality and their analyzes are very dynamic. The instrumentation is easy to assemble and they are small, you can take them anywhere and assemble them with total ease. Its most used application is the qualitative analysis and detection of molecules present in a material
If you want more information about the subject you can visit the following links:
Infrared Spectroscopy: Identifying Functional Groups
APPLICATIONS OF IR SPECTROSCOPY

Video credits @gtg
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Hi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV