So far, I have treated carbonyl compounds together and looked at reactions of the C=O functional group in both aldehydes and ketones. They do, however, differ in their reactions with oxidising and reducing agents. Therefore, I will now look at reactions of the aldehyde functional group separately from those of the ketone functional group.
REDUCTION OF ALDEHYDES AND KETONES
With a reducing agent, such as NaBH4 or LiAlH4 aldehydes give primary alcohols and ketones give secondary alcohols. Notice that the reducing H in the equation is in brackets (to balance the equation simply – it does not represent atomic hydrogen). The reaction is not that simple, as you can see from its mechanism in my previous post. The reduction reactions of aldehydes and ketones are essentially the reverse of the oxidation reactions of primary and secondary alcohols.
OXIDATION OF ALDEHYDES
Aldehydes are prepared by oxidising primary alcohols, and ketones by oxidising secondary alcohols. In the case of primary alcohols, if the aldehyde is not removed from the oxidising agent immediately it is formed, it is further oxidised to give the carboxylic acid. The aldehyde is removed from the reacting mixture by distillation. Primary alcohols are usually mixed with acidified potassium dichromate and the aldehyde distilled off as soon as it is formed to prevent further oxidation. If the primary alcohol is refluxed with K2Cr2O7(aq)/(H+(aq)), then a carboxylic acid results.
Ketones resist further oxidation, because they have no oxidisable hydrogen bonded to the carbonyl group. However, with prolonged heating they can be oxidised by strong oxidising agents. By contrast, the oxidation of aldehydes occurs even in air, so that bottles of opened aldehydes soon contain amounts of carboxylic acids. This difference in the reactivities of aldehydes and ketones to oxidation is one of the main reasons they are considered as separate classes of compounds. It also provides an ideal way to distinguish between them.
Oxidation of aldehydes by acidified potassium dichromate(VI)
When an acidified solution of the orange dichromate(VI) ion is warmed with an aldehyde, it is reduced to the green chromium(III) ion. In this reaction the aldehyde is ethanal.
Oxidation of aldehydes by Fehling’s and Benedict’s solutions
Either or both of these reagents may be used in your chemistry course. They are alkaline solutions that contain copper(II) as complex ions, so the colour of both is blue. The copper(II) ions act as a mild oxidising agent. When the reagent is mixed with an aldehyde and heated, the aldehyde is oxidised to give a carboxylic acid, while the copper(II) ions are reduced to a brick-red precipitate of copper(I) oxide, Cu2O. A balanced equation for this reaction is:
RCHO + 2Cu²+(aq) + 2H2O → RCOOH + Cu2O(s) + 4H+(aq)
This reaction can be used to distinguish between an aldehyde and a ketone. While this reaction is less used now that we have spectrometers, it is still an important test in some situations, such as diagnosing diabetes.
Fehling reaction. FK1954, Public Domain
Tollens’ reagent and the oxidation of aldehydes
Tollens’ reagent provides yet another way to test for aldehydes. This time, the oxidising agent is a complex of silver(I) ions, which are reduced to silver in the test. There is a figure of a silver coating the inside of a test tube, making a ‘silver mirror. The complex, which has the formula [Ag(NH3)2]+, is made by mixing together aqueous solutions of ammonia and silver nitrate. The resulting solution is called Tollens’ reagent, or ammoniacal silver nitrate. When warmed with an aldehyde, it produces the ‘silver mirror’.
This is a simplified balanced equation for the reaction:
RCHO + 2Ag+(aq) + H2O → RCOOH + 2Ag(s) + 2H+
The reaction provides one of the ways in which mirrors are silvered.
SUGARS
Glucose gives a positive test with both Fehling’s and Benedict’s solutions. As it has this reducing property, glucose is known as a reducing sugar. In one of its forms, glucose contains an aldehyde group, which accounts for this property. Diabetes is easily diagnosed using Benedict’s or Fehling’s reagent, which detects glucose in urine samples; and Benedict’s reagent in tablet form, or impregnated into a strip of paper, is used by diabetics to monitor their blood sugar levels.
Alcoholic drinks and the formation of aldehydes
When you have an alcoholic drink, ethanol passes into your bloodstream. The liver has to break down the ethanol. In the first stage, it is oxidised to ethanal. Ethanal is then oxidised into other products. However, if you drink a lot of ethanol in a short space of time, the ethanal that enters the bloodstream is distributed throughout the body. Your face goes red, you may feel an unpleasant tingling in the limbs and nausea, and your blood pressure may drop.
Methanol is added to ethanol that is intended for other uses, to make it unfit for drinking. This mixture is commonly known as methylated spirits (meths). Once inside the body, methanol is oxidised into an aldehyde, which rapidly causes liver damage and blindness. So, unfortunately, if people are determined to drink meths they are risking their lives.
TRI-IODOMETHANE TEST
This is a useful test for the CH3CO group in carbonyl compounds. An alkaline solution of aqueous iodine is warmed with the substance suspected to contain this group. Iodine has a very low solubility in water. Therefore, the aqueous solution is made up by dissolving it in KI solution. If a yellow precipitate of tri-iodomethane is produced, it is highly likely that CH3CO is present.
As I2(aq)/NaOH(aq) is an oxidising agent, the tri-iodomethane test also gives a positive result with alcohols that contain the CH3CH(OH) group.
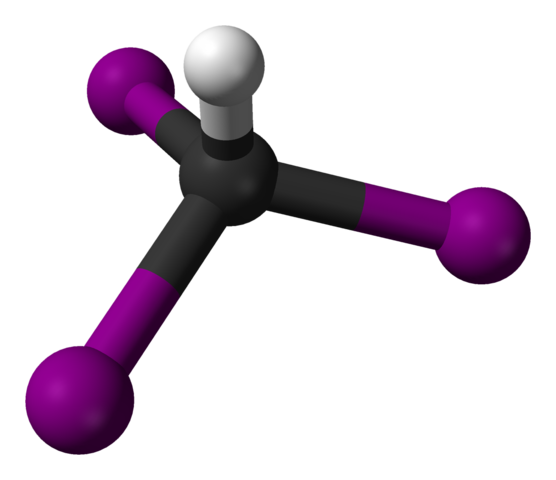
Ball and stick model of iodoform. Ben Mills, Public Domain
SUMMARY
After studying this post and the previous one, you should know the following:
- The carbonyl group C=O is found in both aldehydes (RCHO) and ketones (RCOR’), so they are called carbonyl compounds.
- The polar nature of the carbon-oxygen double bond in the carbonyl group makes it susceptible to nucleophilic addition reactions. This contrasts with the electrophilic addition reactions of the alkene carbon-carbon double bond.
- 2,4-dinitrophenylhydrazine gives characteristic orange precipitates with carbonyl compounds. The resulting 2,4-dinitrophenylhydrazone derivatives can be used to identify specific aldehydes and ketones from their precise melting points.
- Both aldehydes and ketones undergo nucleophilic addition reactions with HCN and with H- ions (from, for example, NaBH4).
- Aldehydes (but not ketones) are oxidised to give carboxylic acids by mild oxidising agents, such as Cr2O72-/H+, alkaline solutions of Cu2+ (Fehling’s solution) and Ag+ (Tollens’ reagent). These reactions can be used to distinguish between aldehydes and ketones.
- Alkaline aqueous iodine gives tri-iodomethane (CHI3) with CH3CO compounds, and also with alcohols that contain CH3CH(OH).
Thanks for reading.
REFERENCES
http://www.differencebetween.net/science/difference-between-aldehydes-and-ketones/
https://en.wikipedia.org/wiki/Carbonyl_reduction
https://www.chemguide.co.uk/organicprops/carbonyls/reduction.html
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch15/ch15-2-6.html
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch17/ch17-3-5-1.html
https://www.chemguide.co.uk/organicprops/alcohols/oxidation.html
https://www.toppr.com/guides/chemistry/aldehydes-ketones-and-carboxylic-acids/oxidation/
https://www.embibe.com/study/oxidation-of-aldehydes-and-ketones-with-benedict-s-solution-concept
https://www.chemguide.co.uk/organicprops/carbonyls/oxidation.html
https://www.youtube.com/watch?v=_0TqG8-N8DI
https://www.chemguide.co.uk/organicprops/carbonyls/oxidation.html
https://www.hindawi.com/journals/ijac/2011/797604/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2872347/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4693266/
https://en.wikipedia.org/wiki/Iodoform
https://chemistryguru.com.sg/triiodomethane-or-iodoform-test

This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.