The mechanism of a reaction details the
smaller steps in the process that results in the formation of the end products
from the starting material. In particular, a mechanism seeks to explain which
bonds are broken and formed and when and how they are broken and formed. Free-radical
substitution is split into three, clearly defined steps:
- Initiation: This is the formation of the free radical.
- Propagation: This involves reactions that maintain the presence of the free radicals.
- Termination: This involves reactions that remove the free radicals.
Initiation
We have already described the formation of a chlorine free radical. Ultraviolet radiation is used to supply the energy to break the chlorine molecule into free radicals as follows:
Cl2 (g) → 2CI• (g)
This equation tends to mislead, since it suggests that all the chlorine molecules are changed into free radicals. In fact, only an extremely small percentage of chlorine molecules are dissociated to form free radicals, and most remain as chlorine molecules.
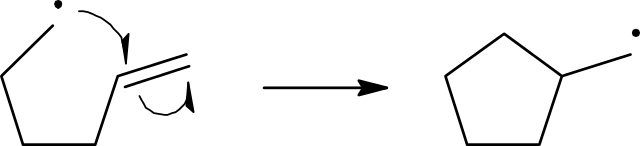
Propagation
Reactions take place when the appropriate particles collide. Since free radicals are so reactive, they almost invariably react with any particle they collide with. In the chlorine free-radical substitution of methane, once the very first free radical is produced, it is most likely to collide with either a chlorine molecule or a methane molecule. The collision with the chlorine molecule does not lead to any change.
Cl•(g) + Cl2(g) → Cl2(g) + Cl•(g)
When the highly reactive free radical collides with a methane molecule, it gains an electron from one of the covalent bonds in the molecule. In this case it is the C–H bond. This gives:
CH4(g) + Cl•(g)→ CH3•(g) + HCI(g)
This reaction produces a new free radical, CH3• which is the methyl free radical. The methyl free radical is also very reactive so as soon as it collides with a particle, it reacts. Which particle it collides with is just a question of chance or probability. The most likely will be the particles that are in the greatest concentration. Certainly, at the start of the reaction, these will be either methane or chlorine molecules:
CH3•(g) + Cl2(g) → CH3CI(g) + Cl•(g)
Notice that the two steps just described regenerate the chlorine free radical, which explains why they are called propagation steps. Generally, two steps can be used in explaining the process as a characteristic reaction of a C–H bond associated with a carbon atom with four single bonds. The product chloromethane still has three hydrogen atoms each bonded to carbon atoms. So, when a chlorine free radical collides with a chloromethane molecule, a reaction will take place:
CH3CI(g) + Cl•(g) →CH2Cl•(g) + HCI(g)
CH2Cl•(g) + Cl2(g) → CH2Cl2(g) + Cl•(g)
Provided there is sufficient chlorine present, this reaction can continue until all the hydrogen atoms have been substituted. The composition of the reaction mixture varies with the time allowed for the reaction and the mole ratio of methane and chlorine.
Termination
The propagation steps cannot go on forever, because eventually chance takes a hand and a free radical collides with another free radical. Although this is not very likely, it is not impossible – particularly when there are many millions of collisions per second. When two free radicals collide, they react and form a covalent bond. As any two free radicals may collide, note that all of the following are possible termination steps.
Cl•(g) + Cl•(g) → Cl2(g)
Cl•(g) + CH2Cl•(g) → CH2CI2(g)
CH3•(g) + CH3•(g) → C2H6(g)
Also note that one of the products is ethane, a molecule that does not even contain chlorine.
FLUORINATION OF ALKANES
Fluorine is a much more reactive halogen than chlorine, and so it should react much more readily with alkanes. The bond dissociation energy for a fluorine molecule is 158 kJ mol-1, considerably smaller than that for a chlorine molecule at 244 kJ mol-1. The reason for this low bond energy is the repulsion experienced by each atom because of the very small bond length of 254 pm in a fluorine molecule. This makes the initiation step for fluorine much easier than that for chlorine.
The reaction of fluorine with alkanes does not need the presence of ultraviolet light and it proceeds much faster than the reaction of chlorine. Consequently, it is difficult to produce monofluoro derivatives of alkanes. The reaction is often explosive and produces the perfluoro derivative (all H atoms are substituted by F atoms). The reaction of fluorine with alkanes is slowed down by diluting the fluorine with nitrogen.
Uses of Organofluorines
Organofluorine compounds occur rarely in nature, but because of the wide range of their applications –from inert solvents in the electronics industry to highly active pharmaceuticals – their development and production are increasingly important. The three-dimensional shape of an organofluorine molecule determines its biological activity. Substitution of a fluorine atom for a hydrogen atom rarely changes the shape since the change in bond length is small, being from 120 pm (120 × 10-12 m) in a C-H bond to 147 pm in a C-F bond. The C-F bond is also very strong and resistant to reaction with water (hydrolysis), which is why pharmaceuticals that contain this bond are liable to remain active in the aqueous medium of a living cell for a long time.
Perfluoro compounds are very unreactive, which has led to yet further applications for which being chemically inert is vital. Perfluorodecalin, for example, dissolves oxygen very efficiently and has been successfully transfused into human volunteers as a blood substitute. Other perfluorohydrocarbons are being developed to provide a fluid that will preserve donor organs before transplantation. There are still problems with these perfluoro compounds, since to be of use they must remain as an emulsion in water. Unfortunately, they tend to separate during storage.
Polytetrafluoroethene (PTFE) is a perfluorinated polymer that is used to make non-stick surfaces, such as on saucepans and frying pans. Coatings are being developed from polymers that include fluoro-substituted alkanes, since these side chains make the surface much easier to clean by their ability to shrug off stains and dirt. Clothes have been developed with such a coating, and it may be possible to make a surface that could remain graffiti free.
BROMINATION OF ALKANES
Bromine is less reactive than chlorine, and the free-radical reaction with alkanes is much slower than with chlorine. This is surprising since the bond energy for a bromine molecule is only 151 kJ mol-1. The reason for this is related to the strength of the carbon-halogen bond that is formed, since a carbon-chlorine bond is much stronger than a carbon-bromine bond, and hence more stable.
When a few drops of liquid bromine are added to hexane, a liquid alkane, the mixture is left in direct sunlight, there is a very slow reaction.
Eventually, the orange colour of the bromine disappears, leaving a mixture of all the structural isomers of monobromoalkane as the main products:
C6H14 (l) + Br2 (l) C,H Br() + HBr(g)
Monobromohexar
FREE-RADICAL SUBSTITUTION AS A SYNTHETIC REACTION
Although free radical substitution is a very successful way of introducing halogen atoms into an alkane molecule, it suffers from a major drawback: often a large variety of products is formed.
The aim of synthesis is to make a compound in high yield and purity, using few reactions and involving cheap starting materials. The large number of products formed during free-radical substitution makes it a poor tool in organic synthesis.
The only way to make the reaction useful is to minimise the number of possible products, so that a relatively high yield of the required product is obtained. In practice, this means using reactants that have very few hydrogen atoms to be substituted.
Free-radical chlorination in carboxylic acids and methylbenzene
SUBSTITUTION IN METHYLBENZENE
Methylbenzene has three C-H bonds not attached to the benzene ring; these hydrogen atoms are chemically identical and behave in a similar way to the hydrogen atoms in methane. Therefore, it is quite easy to synthesise chloromethylbenzene by passing chlorine into boiling methylbenzene in the presence of ultraviolet light. By keeping the available chlorine concentration low, the monosubstituted product chloromethylbenzene acid is the major product.
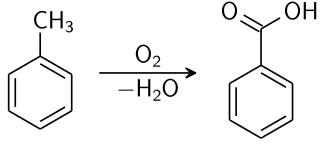
Benzoic acid chemical synthesis. Krishnavedala, Public Domain
SYNTHESIS OF THE AMINO ACID GLYCINE
Ethanoic acid also contains three hydrogen atoms that are similar to those found in methane and so ethanoic acid can be chlorinated to give chloroethanoic acid: Amino acids are the building blocks of proteins. Glycine (aminoethanoic acid) is the simplest amino acid. It has a two-carbon skeleton, so ethanoic acid is an ideal candidate for its synthesis.

In preparing halogenoalkanes by the halogenation of alcohols: monohalogenoalkanes are best prepared by replacing the hydroxyl group in an alcohol with a halogen atom. A variety of reagents can be used for conversion such as the concentrated hydrochloric acid, phosphorus(V) chloride, concentrated hydrobromic acid, phosphorus(III) bromide, and red phosphorus and iodine.
Thanks for reading.
REFERENCES
https://www.britannica.com/science/reaction-mechanism
https://opentextbc.ca/chemistry/chapter/12-6-reaction-mechanisms/
https://en.wikipedia.org/wiki/Reaction_mechanism
https://www.scripps.edu/baran/images/grpmtgpdf/Su_May_08.pdf
https://pubs.acs.org/doi/abs/10.1021/jo00381a017
https://socratic.org/questions/why-is-the-reaction-of-alkanes-with-fluorine-is-difficult-to-control
https://www.sciencedirect.com/book/9780444537485/organofluorine-compounds-in-biology-and-medicine
https://www.sciencedirect.com/topics/chemistry/organofluorine-compound
https://en.wikipedia.org/wiki/Organofluorine_chemistry
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch04/ch4-4-4.html
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alkanes/Reactivity_of_Alkanes/Halogenation_of_Alkanes
https://en.wikipedia.org/wiki/Radical_substitution
https://en.wikipedia.org/wiki/Free-radical_reaction
http://www.docbrown.info/page06/OrgMechs4a.htm
https://www.chemguide.co.uk/mechanisms/freerad/tolueneandcl2.html
@tipu curate 2
Posted using Partiko Android
Upvoted 👌 (Mana: 0/15 - need recharge?)
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
I must have learned that in the first year of medicine But it seems So far in my memory.
It's a very good article and it's very interesting
Posted using Partiko iOS
Hello, @debo-medstudent.
Thanks for coming by and also for the nice comments.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.