Pyrolysis
Pyrolysis is called a type of chemical reaction in which a chemical compound or, more commonly, a material product of the complex association of compounds (wood, plastics) is subjected to the action of heat ( and only of the heat) and for that effect, decomposition products are produced.
Pyrolysis reactions are a particular case of chemical reactions called thermolysis (heat rupture, also called cracking or cracking, a word that comes from the English verb to crack which means to crack or crack and is usually interpreted as equivalent to breaking). The one that breaks is the molecule subjected to the action of heat, the process that leads to that break is pyrolysis and the one that pyrolyzes is the molecule, in this case without combining with other products, only by the action of heat.
Pyrolysis process
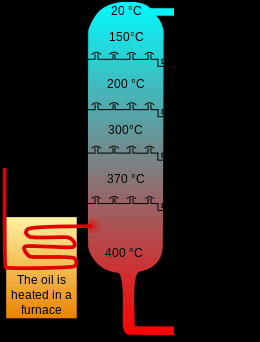
The process of pyrolysis heats the materials up to 400-500 degrees in an environment without oxygen, causing a thermostatically reaction of the materials used. The best-known use is for the creation of charcoal. The absence of oxygen prevents combustion. High temperatures cause the separation of gas and oil from the materials. This chemical process generates heat. Therefore, the process produces enough thermal energy to sustain itself. The process lasts a few minutes. Generates variable amounts of gas, oil, and coal.
Since pyrolysis is a chemical process, the process is fast and reliable. In all processes of coal utilization and conversion, some type of pyrolysis takes place. For example, gasification is related to pyrolysis. Pyrolysis is a very old technique, dating from the 18th century, where the separation of hydrocarbon fuels was already allowed.

Stages of the process
The process consists of 3 clearly differentiated stages:
1 In this first stage, slow decomposition occurs with the production of small amounts of water, carbon oxides, hydrogen, and methane. This is a consequence of the breaking of links due to the high temperature at which the process is carried out and also the consequence of the release of gases retained in the coal.
2 This second stage is known as active thermal decomposition. The temperature increases and a deeper fragmentation of the carbon molecule occurs with the formation of condensation hydrocarbons and tars. This stage begins around 360 C and ends when temperatures have reached around 560 C.
3 The last stage, which takes place at temperatures above 600 C, is characterized by the gradual elimination of hydrogen and other hetero atoms.
Products from pyrolysis
Pyrolysis is a very significant and important coal conversion technique since it allows a diversification of its uses, from obtaining basic products for the industry to the production of liquid fuels.
Coke
It is the most important product of pyrolysis. It is used as a raw material that feeds blast furnaces for the production of steel. It is a porous and very reactive carbon. A coke oven is a series of prismatic boxes attached to each other that work in semi continuous. The coal is placed in alternative boxes. Among them, burners are placed where the gas produced in the pyrolysis is burned. The gas provides the necessary heat for the process. The coal is charged and a time is left during which the pyrolysis takes place. Once the coke is produced, it is discharged and reloaded. The gases and liquids are extracted from above, condensing the liquids.
Gas
The gas produced in the coke is fed to a vapor recovery unit, where liquefied petroleum gas (LPG) and refinery fuel gas are produced. The LGP gas is treated with sulfuric acid and fuel gas and propane and burano are obtained, the LPG can be used as raw material for the alkylation or the polymerization unit.
Naphtha
The light coke naphtha, after stabilization in the steam recovery unit, is often sweetened to reduce mercaptans and then sent to the gasoline pool. The heavy naphtha is hydro treated and is used either as a raw material of the catalytic reformer or directly sent to the gasoline pool.
Diesel oil
The coking light gas oil can be treated with hydrogen for color stabilization and used in the refinery as heating fuel. Heavy gas oil is commonly used as a raw material in the catalytic cracker or hydro cracker.
