Introduction
From a biochemical point of view, Sustenance of life is quite a major complex process which involves a whole lot of other minor processes.
Food is essential for survival. But how exactly is food utilized in the maintenance of survival ? You are told that rice contains carbohydrates but has it ever occurred to you how your body can actually obtain this carbohydrate from rice ? Well, you are told that the body absorbs food but how do cells utilize this food ?
You do not eat for two days but then you manage to stay alive. You are told your brain can only utilize glucose but what happens during prolonged starvation where all carbohydrate stores are literally depleted. Do you go insane ? Do you develop neuronal disorders ? Well no. Something happens and I’ll tell you that on the long run.
There’s a whole lot of questions to be answered and I’ll dedicate this series to carefully and simply explain cellular processes that occur which are ultimately necessary for survival.
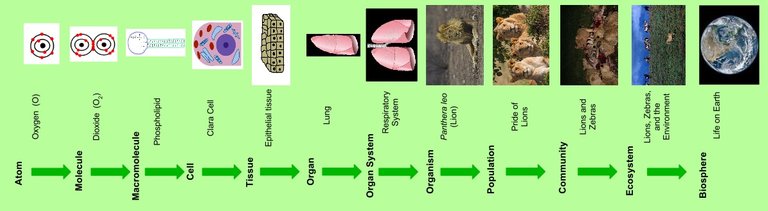
Biochemistry is basically the study of life at the cellular level. Looking at the levels of organization
Cell < Tissue < Organ < System
it shows that the cell is the smallest functional unit of life. But there are even molecules which are quite smaller than cells and can be found in these cells. An example is the DNA.
DNA itself is made up of even smaller molecules known as nucleotides. Nucleotides are made up of even smaller molecules (nucleobase, ribose Sugar and phosphate group). The nucleobase is made up of even smaller substances known as atoms (carbon, nitrogen, hydrogen, oxygen). The ribose Sugar (ribose is five carbon sugar. Just like glucose which is a six carbon sugar) is made up of even smaller atoms (carbon, oxygen, hydrogen). So it’s basically just a matter of different levels of organization.
To properly begin this series, I’ll like to introduce a term well used by biochemists. This term is biomolecules
What is a biomolecule ?
A biomolecule is to a biochemist what an atom and an element are to a physicist and a chemist respectively.
A biomolecule, otherwise known in full as a biological molecule is a term used to describe all molecules and ions found in the biological system that are essential for biological processes like cell division, cell morphogenesis and cell development. They are integral parts of metabolic pathways. They are most often ends to means but can also be means to ends. This means most metabolic pathways aim at producing them (end products) and they can also act as starter or intermediate molecules in the production of other metabolites.
The biomolecules are what we throw around everyday in our day to day conversations without even knowing they are in fact called “biomolecules”. The four main biomolecules in biochemistry are;
- Carbohydrates
- Lipids
- Amino acids
- Nucleic acids
Carbohydrates
To a number of people, carbohydrates are energy giving foods. Considering the number of ATPs (unit of storing energy) generated as a result of the breakdown of a glucose molecule, that is technically correct.
The biochemical definition is quite different. Carbohydrates can be defined as polyhydroxyl aldehydes or ketones consisting of hydrogen, carbon and oxygen atoms. They have a general formula, Cm (H2O)n. They are divided into simple and complex carbohydrates.
Simple Carbohydrates
The simple carbohydrates consist of the monosaccharides, disaccharides and the oligosaccharides.
Monosaccharides
They are the simplest carbohydrates and are simply known as sugars. They are made up of one sugar unit. They are the building blocks of disaccharides and the complex carbohydrates.
They are easily absorbed by the body. Matter of fact, all carbohydrates are ultimately broken down to glucose while the monosaccharide counterparts (fructose, mannose, galactose) are converted into glucose to be easily absorbed by the body.
Monosaccharides cannot be further broken down into simpler compounds. They are generally sweet , soluble in water and take on solid crystalline forms.
They have the general formula CnH2nOn.
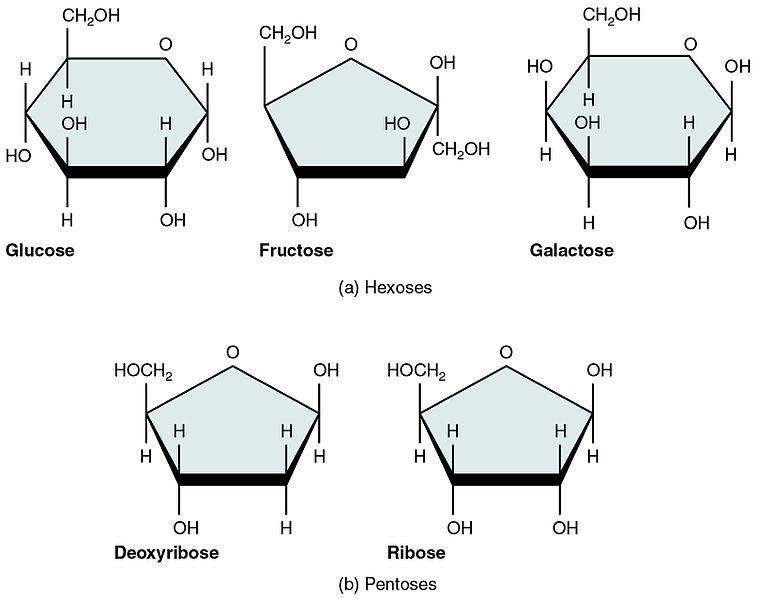
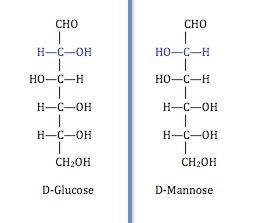
The three common monosaccharides are; Glucose, Fructose and Galactose Monosaccharides are known to exhibit chirality. They can exist in different forms possessing different physical and chemical properties although having the same number of carbons.
Perfect examples are glucose and galactose which have the same number of carbon but they differ slightly in arrangement at carbon number 4. They are generally considered epimers (molecules different in configuration around a particular carbon atom).
Classification of Monosaccharides:
Monosaccharides can be classified based on the number of carbon atoms they possess
Trioses are three carbon sugars. Examples are glyceraldehyde and dihydroxyacetone. They are very important in metabolic pathways like glycolysis (a pathway that breaks down glucose) as well as Triacylglycerol synthesis. This will be shown in the upcoming series
Tetroses are four carbon sugars. Examples are erythrose and erythrulose. They are involved in the shikimate pathway which is a pathway that leads to the synthesis of the only three essential amino acids; Tryptophan, phenylalanine and tyrosine (all part of the upcoming series) ;)
Pentoses are the five carbon sugars. Examples are ribose and ribulose. Ribose is a very important constituent of nucleotides. DNA, being an example. Ribulose is a very important intermediate in the pentose phosphate pathway which is an alternative pathway for glucose breakdown.
Hexoses are the six carbon sugars. We happen to be vary familiar with these ones. Glucose and Fructose are the popular examples here. Glucose is the starting substrate in glycolysis while it is the end product in gluconeogenesis (a pathway that produces glucose from non-carbohydrate precursors. Fructose is also a constituent of semen.
Heptoses are the seven carbon sugars. An example is sedoheptulose. It is an intermediate in the pentose phosphate pathway.
Monosaccharides can also be classified based on functional groups;
Ketoses contain a ketone group. Examples include; Fructose, dihydroxyacetone, erythrulose. These sugars posses a ~C=O group.
Aldoses contain an aldehyde (~CHO) group. Examples include; glucose, glyceraldehyde, ribose and erythrose.
Epimers, Anomers and Stereoisomers
Sugars can possess the same number of carbon atoms yet exist in many different forms. That has been the reason behind the craszy names you see above but well, you have me to explain exactly what they mean.
Epimers.
There are sugars that have the same number of carbon atoms but then, differ around a certain carbon atom (chiral carbon) and as a result their physical and chemical properties are altered. Those sugars are known as epimers. Galactose is considerably a C4 (carbon 4) epimer of glucose cause it differs in arrangement at carbon 4 compared to glucose. Mannose is considered a C2 epimer of glucose and well you get the story by now.
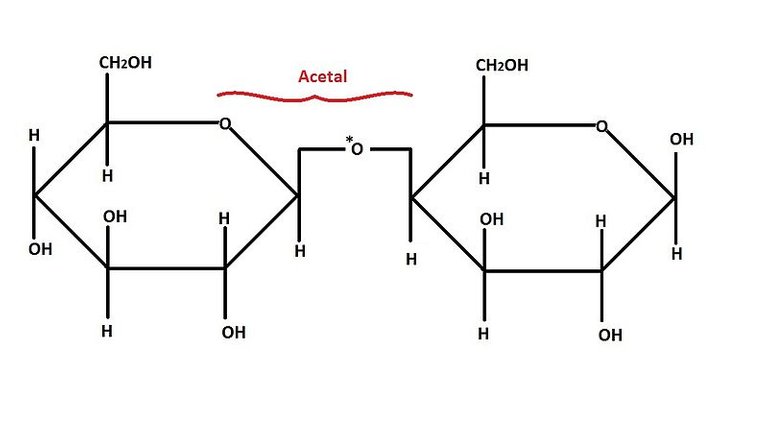
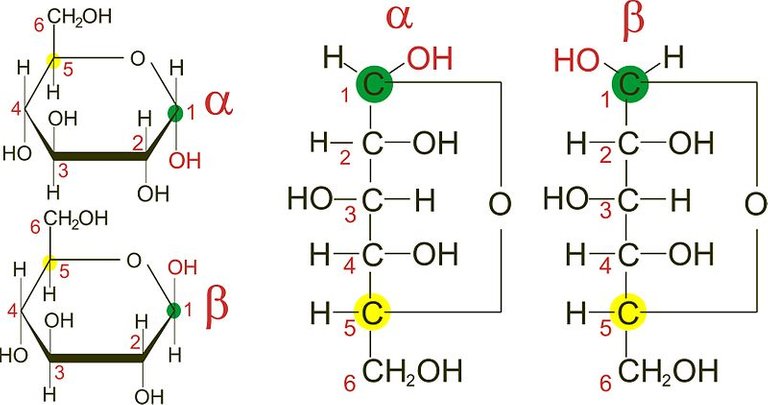
Anomers
These are sugars that differ at the hemiacetal or acetal carbon. (Hemiacetal carbon is the carbon which has an alcohol group(~OH) and an aldehyde group (~CHO) attached to it while the acetal carbon has that hydroxyl group (~OH) replaced by an organic fragment (an acetal group) in addition to an aldehyde group). The anomeric carbon is usually carbon 1 (C1) and changes in its configuration gives rise to anomers Examples are the alpha and beta D-glucose which differ only at carbon 1.
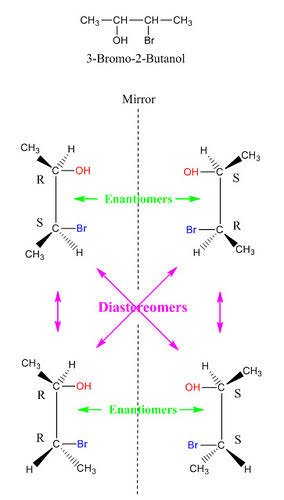
Stereoisomers
Stereoisomers are molecules that have similar molecular formulas but different 3-D orientations of their atoms in space. They comprise enantiomers and diastereomers.
Enantiomers are basically different forms of the same molecule which present as mirror images of each other. Our hands are perfect examples of enantiomers.
Enantiomers tend to have similar physical properties but rotate plane polarized light in different directions.
The common Enantiomers that can be seen in nature are; L and D- amino acids. L and D- glucose. It’s pertinent to know that only one of these Enantiomers are generally stable and prevalent at the end of the day. For amino acids, the L- form is the most stable and prevalent. For glucose, the D- form is the most stable and prevalent.
Diastereomers, for a start, are stereoisomers that are not mirror images of each other. Unlike the enantiomers, they rarely have the same physical properties. They are basically stereoisomers that differ at their stereocenters. Epimers are diasteromers. They differ at only one stereocenter.
Roles of Monosaccharides in nature
- Glucose is major source of energy
- Lyxose is found as a component of lyxoflavin in the human heart.
- Ribose is a vital component of DNA and RNA
Disaccharides
Disaccharides are those carbohydrates which are made up of two monosaccharide units. The two Monosaccharides units are held together by a special kind of bond known as glycosidic linkage.
They are water soluble molecules just like the monosaccharides.
The three most abundant saccharides are; sucrose, lactose and maltose. They are composed of different monosaccharide units.
- Sucrose is composed of one glucose unit and one Fructose unit.
- Lactose is composed of one glucose unit and one galactose unit.
- Maltose is composed of two glucose units.
Enzymes can break down these disaccharides into their respective monosaccharide units. These enzymes are called Disaccharidases (sucrase acts on sucrose, maltase acts on maltose and lactase acts on lactose).
Classification of disaccharides
Disaccharides are classified into;
- Reducing disaccharides
- Non-reducing disaccharides
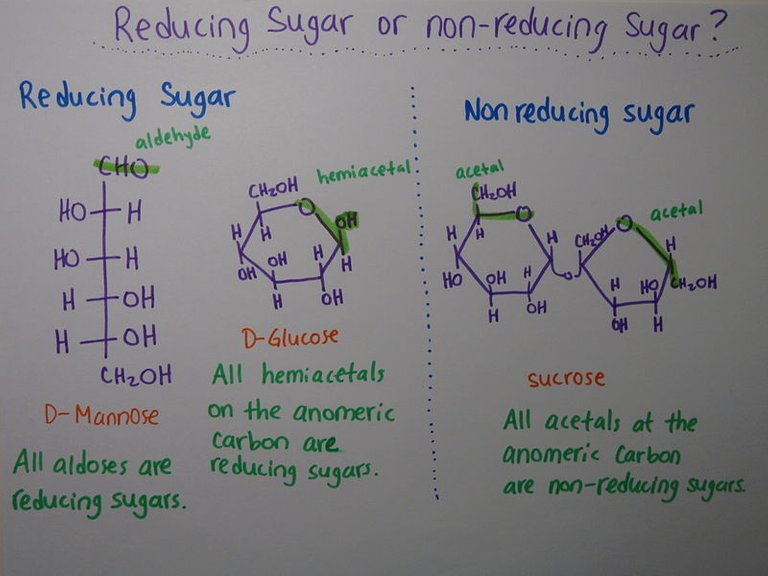
Reducing Disaccharides:
They contain a free hemiacetal end in one of their monosaccharide units and thus can act as reducing agents. An example is maltose.
Non-reducing Disaccharides:
The anomeric centers of their monosaccharide constituents are connected through an acetal linkage and so there are no free hemiacetal ends and thus, the disaccharide cannot act as a reducing agent. Suitable examples of reducing disaccharides here are sucrose and trehalose (composed of two glucose units).
- the reducing property of glucose makes its identification in solution and substances quite easy. As it can reduce certain agents while being oxidized in the process. This is a very important basis of colorimetric reactions (reactions that produce colour).
Summary
Biomolecules are essential molecules in the biological system. They constantly take part in anabolic (building up) and catabolic (breaking down) reactions leading to the formation of products as well as the release or take up of energy. They consist of carbohydrates, amino acids, lipids and nucleic acids. Their importance in nature cannot be overestimated and thus, they remain relevant in the sustenance of life.
Thank you for reading and watch out for my next series on the chemistry and functions of polysaccharides and also another topic you’ll have to find out yourself ;)
References
Biomolecule
Metabolism
Carbohydrates
Monosaccharides
Epimers
Hemiacetal
acetal
Diastereomer
Enantiomer
Disaccharide
Image Sources
All images are from flickr and wikicommons licensed under creative commons and eligible for commercial use.
I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here

I did a little of biochemistry in school and this refreshed my memory on biomecules. Nice post man!
Thank you very much. I’m glad you found it helpful
Congratulations!,@kingabesh Your post has been upvoted by @reachout via the
nigeriatagOur goal is to support Nigerian minnows on Steemit. Join our discord group https://discord.gg/NWAkKfn
Proudly sponsored via SP donation from @eturnerx , @rufans & @solomon158
Upvotes Benefactor : @bleepcoin & the rest of us
###### Join Our Trail here: https://steemauto.com/dash.php?i=15&id=1&user=reachout
Curator On Duty: Richie, the Manual Bot (BETA)
## Also,We'd like invite you to the @eoscafe discord community https://discord.gg/wdUnCdE , be part of something great
Educative piece I must say. But i saw a little thing that caught my attention:
Glucose is not a major form of energy but rather a source of energy Whose quick metabolism yields ATP, the energy unit of organism
Ermm, can you guys chat on discord?
@vanessahampton, replying here is not a bad thing. Others may get to learn a thing or two. Thank you.
Ok, thanks.
My apologies for butting in @gentleshaid.
Wow an unintentional mistake man. I’ll address that now. Thank you for reading through and ah, I’m still waiting to hear from you on that collab ;) @gentleshaid
i thought as much. I will chat you up about it once i'm a bit free
No problem :)
Told you the right audience will read. Now we are being introduced to biochemistry.
I recall using right and left shoe legs to understand enantiomers.
Well done.
Haha thank you Vanessa. I hope you enjoyed this piece :)
People who liked this post also liked:
Lazzarus-THE POEM by @jegede
FLAMMABLE ICE BUBBLES by @bafspotlight
ATTITIDE OF POSITIVITY by @ifeoluwa88
Hello! I find your post valuable for the wafrica community! Thanks for the great post! @wafrica is now following you! ALWAYs follow @wafrica and use the wafrica tag!
Great article! Well done!
Thank you @davidrhodes124 :)
Once I was live without eating a single bit of food. Even I was curious how this is possible and your article solve my mystery.
Even I experienced that after 7 day of starvation I feel like that I can stay like this for years. Just need water and 2 cup tea.
Very interesting Topic you choose.
Thank you ! Glad you found it interesting
A great article, well done @kingabesh!
Thanks Kate ! :)
😱😱 all these chemistry really confuses me.. I wish they were more simpler to me the way they are to you.
But i was able to pick up few things about bio molecules in you conclusion. Turns out they are essential for anabolism and catabolism.
Lol sorry man. I’m glad you were able to learn a thing or two.
Cheers !
Mehn, no wonder I don't like chemistry! Many terms combined with biological ones to hold in.. I am blown by your effort...
I got to learn alot about the carbohydrate Brothers and some terms too (hopefully I won't forget soon)
Mr King Kong..
Thanks a lot man
You're welcomed