Numerous organic compounds and some inorganic ones exhibit this property and it has implications in chemical especially pharmaceutical industries. Hence, we would be taking a tour through the context of occurrence of chemical compound called optical isomers. Starting from the basis…..
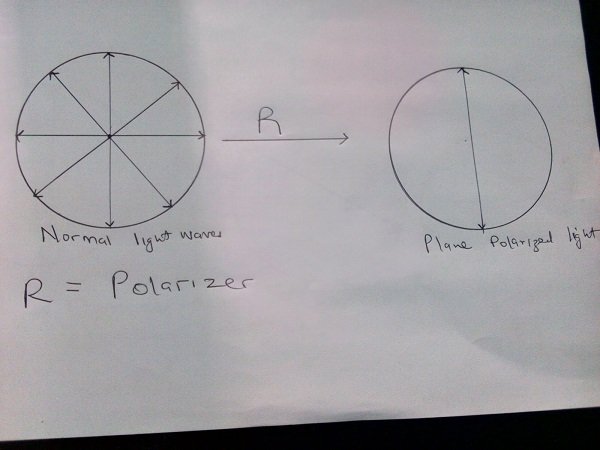
Plane of Polarized light
Light waves vibrate at right angles to the direction of the propagation of light. Many planes pass through the line of propagation, with normal light vibrating in all the planes. It is possible for light in all the other planes to be blocked out leaving vibrations on a single plane, when this happens, the light is said to be plane polarized. Substances which convert normal light to plane of polarized light are known as polarizers we are going somewhere
Optically Active Compounds
Some chemical substances have the ability to rotate this plane polarized light. When plane polarized light is incident on a solution of chemical substance, if the solution rotates the plane of polarized light, it is said to be optically active. Each pure form of a substance has specific rotation (α) which is a measure of the optical activity. The Optical activity could be measured by an instrument known as a Polarimeter. Optically active substances can rotate plane polarized light to the left or right.
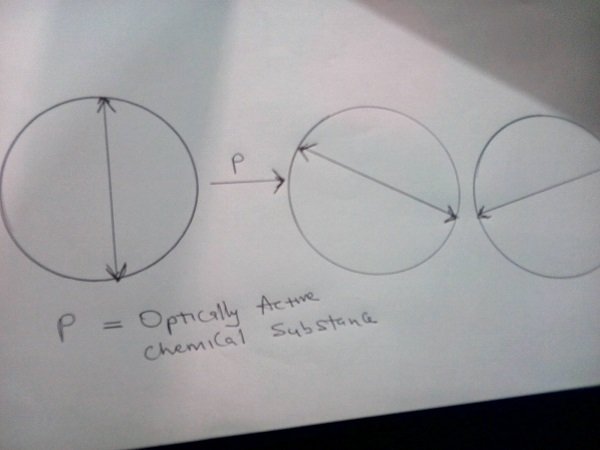
Compounds which rotate plane polarized light to the right are known as Dextrorotatory and the rotation is said to be positive (+), while those which rotate to the left are referred to as Laevorotatory and the rotation is negative (-).
Optical Isomers (Mirror Images)
Chemical compounds which exhibit this property are called optical isomers we are there now. Optical isomers are chemical compounds which rotate plane polarized light with equal magnitude but towards opposite directions (left or right). They are also known as mirror images or Enantiomers. The two forms though identical with the same molecular formula, structural formula, spatial arrangement, connectivity, physical and chemical properties are not superimposable just like our hands and feet. What about our eyes are they superimposable?

I’ll like to read @Mobbs comment on that.
Mixture of equal amounts of each enantiomeric form present together at the same time would give zero rotation (positive cancels out the negative). This mixture is referred to as Racemic mixture or Racemate and it is not optically active. This type of mixture is difficult to separate except through special technique referred to as Chiral resolution. Racemate differs from each separate, pure enantiomeric forms in physical properties like boiling point, melting point, refractive index and so on.
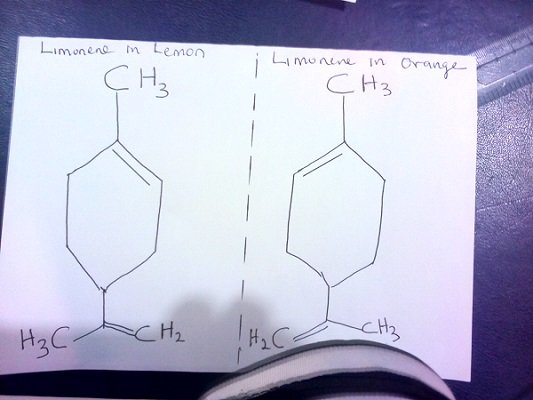
Examples of Optical isomers (chemical mirror images)
The different flavours of orange and lemon is due to the mirror images (optical isomers) of limonene present in them.

Implications
When extracted from natural sources, substances show optical activities that is, they exist in their pure enantiomeric form, for instance lactic acid from sour milk, limonene from orange juice. However, when prepared in the laboratory, they show no optical activity. This is because the product of most laboratory preparation is the racemate form. This product could then be separated into pure separate enantiomeric forms using chiral resolution.
Why is it necessary to separate or differentiate the enantiomeric forms?
There are times when mirror images of optically active compounds behave like the ‘evil twin’. One could be good and beneficial while the other may be bad and detrimental to health. Hence, if prepared through chemical synthesis, they would be produced together hence the need for chiral resolution to separate the racemate into separate pure enantiomeric forms or to use an aalternaative synthetic route known as asymmetric synthesis which would yield only a pure enantiomeric form.
A tragic story
I once read about a tragic story of a woman in the 1960s. A drug, thalidamide was used for treating nausea (morning sickness) in pregnant women especially in the first trimester (first three months of pregnancy). Unknown to the scientists then, this drug has an enantiomeric form which was sold to the pregnant woman. One form was safe and effective (good twin) the other was aa powerful foetus deformer (evil twin). The nausea stopped alright, but at the time of delivery, the woman gave birth to a deformed baby.
Hence, it is necessary to be enlightened about the implications of optical isomerism in the chemical industry especially in drug synthesis.
References
https://en.wikibooks.org/wiki/Organic_Chemistry/Chirality/Optical_activity
Chemistry in Context by Graham Hills and John Holman fifth Edition
)
)
Hello @conas, though am not a chemist, but the way you portray optical isomerism in pharmaceutical industry, is quiet understanding. The case of the pregnant woman is really pathetic. Well it's good that research is advancing every time. Imagine if pharmaceutical industry is still making same mistake today.
Thanks @steep2308, this is one of the good sides of technological advancement.
Weldone @conas for this wonderful article. I really like your illustrations. I can simply say that all our body parts that are in pairs are not superimposable, they are a mirror image to each other. This must be an aspect of physical chemistry because it is pure physics you discussed here. Thanks, @conas for this educative post.
@oluwabori, thanks for your comment. it is actually an aspect of organic chemistry.
You gotta zoom in MUCH closer than that =P
This is an informative post about optical isomerism @conas .
Am getting to know that this is what is responsible for different taste in Orange and lemon.
It is just obe of the occurences, you 'll be suorised at the various occurences and their implications. Thanks for stopping by.
It is just one of the occurences, you 'll be suorised at the various occurences and their implications. Thanks for stopping by.
Congratulations @conas! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Do not miss the last post from @steemitboard!
Participate in the SteemitBoard World Cup Contest!
Collect World Cup badges and win free SBD
Support the Gold Sponsors of the contest: @good-karma and @lukestokes
Hi @conas!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV