It was one of those cold winter morning, George’s mom, Mrs. Price had overslept, she picked up her phone and saw the time was 7:40 am, she had to be at work by 8:00 am, she jumped off her bed and yelled George!!! George was on holiday so he had nowhere to be that morning, why didn't you wake me up she asked? I didn't know you were still asleep the 14 year old boy replied, boil me some water she said and make me a cup of coffee, yes mum he said, she rushed into the bathroom, had her bath and in 10 minutes she was out, it should have taken longer than that but she could not afford to be later than she already was.
She got out and asked George for her coffee, with a smile on his face he handed the coffee over to his mom, he was satisfied knowing he had just helped his mom that morning, she took the coffee from him but then she exclaimed, George Anderson Price she said with a look of bewilderment on her face, I told you to boil the water, didn't I? why in God’s name is this coffee cold as a dog’s nose she asked, ma I did what you asked, I boiled the water then made the coffee George said, she raised her hands making a gesture as if she'd hit the boy, boy don't make me smack your face into the past she said just like a black woman would, but ma, I boiled the water just like you asked he said, urgghhrrr she groaned, disappointed in her son she left for work, she couldn't afford to be later than she already was.
Throughout the day at work Mrs. Price couldn't stop thinking about what George had done, she couldn't believe he lied, she taught him better than that, she most definitely taught him to be smarter than lying about something so obvious, the water was cold, how was it boiled then? So she got home that evening and called her son, boy come here and explain yourself she said, George still kept telling his mom he boiled the water, but why was it cold she asked, so he proceeded to show her what he did.
First he got a beaker he once bought for an experiment from the kitchen, then he went into the garage and got a vacuum pump belonging to his dad, he then went back and filled the beaker with some water from the kitchen, sealed the beaker with a rubber seal and made a small hole on the seal big enough to squeeze the hose from the vacuum pump inside without letting any air into the beaker, he then switched on the vacuum pump while his mother watched him curiously waiting for the magic the little boy was going to perform, after about a minute the water in the beaker started bubbling showing clear signs of boiling as the water was also changing from the liquid to water vapor, Mrs. Price was stunned by this cause not only was the water boiling indeed without the application of any kind of heat, the water also seemed to be getting colder, George looked at his mother, she looked dumbfounded by this demonstration, she looked back at her son and asked how is this happening George? So little George explained how water can boil without the addition of heat to his mum and below is exactly how he explained it.
What keeps water in a liquid, solid or gaseous state?
Just before little George explained how he made water boil without the addition of heat, he first had to explain the 3 phases of water to his mum and why it is found in any of the three states.
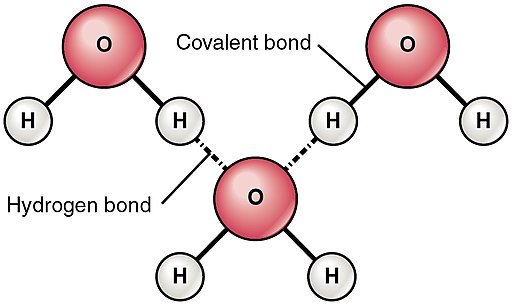
What makes up an individual water molecule? He asked, of course that was a rhetorical question as he proceeded to answer it himself just before his mum could, two hydrogen atoms and one oxygen atom he said, and then asked again, how do these atoms stick together to form water molecules? This time his mom didn’t bother trying to answer as she knows what comes next, you see mama he said, the two hydrogen atoms bond to an oxygen atom by a type of bond called covalent bonds, now the oxygen atom are in water molecules are electro-negative, meaning that they usually carry extra electrons, this is because oxygen’s valence shell has six electrons, so to fill it up it needs to attract 2 more electrons and therefore the electrons in water molecules tend to spend more time around the oxygen atom as it attracts the shared electrons in the covalent bond while it keeps them away from the hydrogen atom (greedy oxygen), this makes the oxygen atom negatively charged while the hydrogen atoms are positively charged, for this reason water is said to be a polar molecule due to its polar covalent bond (different charges on either side).
Thanks to this polarity of water (difference in charge between the atoms in water molecules), individual water molecule can bond to another individual water molecule through a chemical bonding known as hydrogen bonds, this is as a result of the rule which says opposite charges are attracted to each other, so the negative end of one water molecule is attracted to the positive end of another water molecule resulting in a chain of several water molecules bonding to form liquid water.
Hydrogen bonds however are normally weak, this is as a result of the slightly positive charge of the hydrogen atom bonding to the slightly negative charge of the oxygen atom, this results in a weak type of bond which can easily be broken when the individual molecules move further from each other in the liquid state. Water would remain in its liquid state if these bonds remain intact and this is what happens most of the time at temperatures between 0 - 100oC because the weak hydrogen bonds can still hold water molecules together in this situation.
Water can however change its state from liquid to solid or liquid to gas and vice versa, even at room temperature, water molecules are always restless, always on the move, so an increase or decrease in the energy with which they move would result in a change of state, for example water would change from its liquid phase to a solid phase if the energy with which it moves is reduced significantly, this would bring the individual water molecules closer to each other resulting in a stronger bond between them, It can also be changed from the liquid phase to gaseous state simply by increasing the energy with which it moves and thereby driving the individual water molecules farther apart to a point where they are totally free from each other and escape their hydrogen bonds.
With that being said, George went ahead and asked his mum the supposedly simple question.
What is boiling?
This time he fully expected an answer, the long pause made his mum realize it wasn’t rhetorical this time so she said and I quote “well boiling is a process where water is made hot enough to the point where it starts evaporating” George looked at her and said, well you are close but yet not close enough so he decided to get help from this source to help define it better and he said;
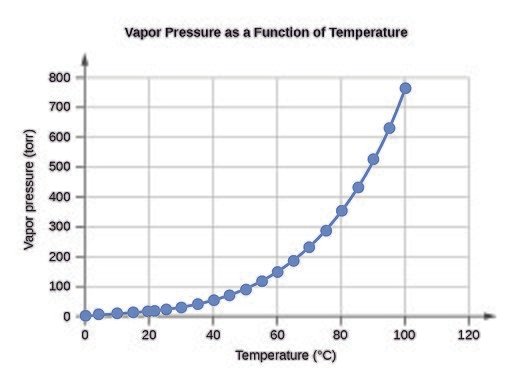
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere.
Haha, Mrs. Price exclaimed and repeated the words “when a liquid is heated” laying emphasis to the verb “heated”, George smiled and said I’m not surprised at this, just like you, a lot of people tend to think boiling is all about heat, but the thing is, heat isn’t the only player when it comes to boiling, as a matter of fact it’s not the most important player as just as I have demonstrated, it can totally be ignored. The most important player when it comes to boiling though is pressure, and just like the definition said, the boiling point of a liquid is the temperature at which the vapor pressure of said liquid is equal to its air pressure and while of course this point can be reached by the addition of heat to a liquid, it’s not the only way.
Pressure: The MVP in boiling
Under normal atmospheric pressure of 101.325 Kpa, the vapor pressure of water would be less than this, so in order to make water boil and vaporize, these two pressures needs to be equal and yes this can be achieved by heating the water up as this would gradually increase the water’s vapor pressure, and when it gets to the point where its equal to the air pressure, individual water molecules would have had the kinetic energy required to escape their hydrogen bonds and vaporize.
A better way to visualize this is to think of the three states of water as three adjoining rooms, the middle room being the liquid phase while the first room is the solid phase and the third room as the gas phase, then think of water has a man constantly trying to move to the third room, but has its movement restricted by the floor of the room which is like a treadmill restricting the man’s movement, in order for the man to move to the third room from the second room, he needs to overcome the resistance from the treadmill and to do that he needs to have some type of energy that helps him increase his speed cause any reduction in his speed would also send him back to the first room, the solid room.
The energy this water man requires can be thought of as heat, heat him up and he is pumped, he can run faster than the treadmill and move to the gas room which is the third room but while heating the man up can most definitely get him to the third room, we can be also get the man to the other room another way.
Note: 780torr = 101.325kpa
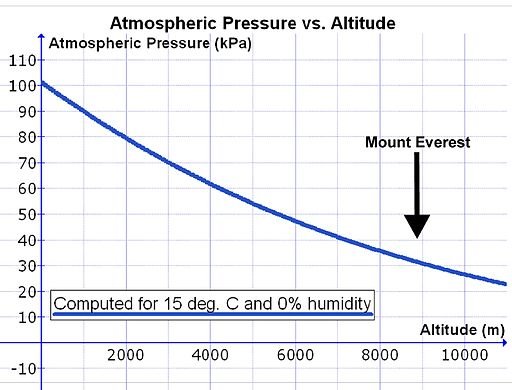
Now think of the treadmill as the air pressure, if we want to get the man into the other room but we don’t want to go through the process of burning fuel and contributing to the excess carbon already in the atmosphere, why don’t we just reduce the speed of the treadmill to a point where it’s even slower than the man’s speed, this would definitely get Mr. water safely into the third room with no heat added, so by simply bringing down the atmospheric pressure surrounding the water to a point where its equal to the water’s vapor pressure, water would definitely be made to boil without the addition of heat and this is what little George did, by sealing of the beaker with the rubber seal, he prevented air from getting inside and then he used the vacuum pump to remove the air already present in the beaker and thus lowering the air pressure in the water and its gets more interesting, if the atmospheric pressure is continuously lowered, the water temperature would actually drop gradually till it gets to a point where water would freeze and then you can say you’ve boiled water to a freeze, this is possible because when water boils this way, the individual water molecules with more kinetic energy have higher temperature and are vaporized, the rest of the water molecules present would have less kinetic energy and lesser temperature and they are therefore colder, when this process is continued the water molecules evaporating takes away the water heat leaving the remaining water much colder as a result
This little technicality is responsible for why tea in an airplane sucks and why water can boil faster on mountain tops than on sea level, this is due to the fact that the atmospheric pressure decreases as we go higher, which means that lesser temperature is required to boil water than the 100oC required to boil water on sea level and while airplanes are always pressurized, they are not fully pressurized, only about 75% pressurized, so boiling water on airplanes is easier but it doesn’t mean the water would be hot enough to make a good tea and that’s why the tea you had on that airplane the last time you traveled sucked mama George said.
Hmmm, this is interesting Mrs. Price said, did you discover this on your own son, because if you did we can probably win a Nobel price from this, George laughed and said, sadly mom the answer is no, this is well known in industries such as the water industry and the food industry, I can tell you all about it if you want ma, go ahead son, I’d like to hear all about it Mrs. Price said so George continued.
Application of low pressure boiling in the water industry
In the water industry, this concept is undesirable particularly when it occurs in pumps but it’s found some useful application in the area of waste water treatment.
- Cavitation in pumps: the undesirable aspect of low pressure boiling in the water industry comes in the form of cavitation in pumps, the majority of pumps used in the industry are centrifugal pumps, specifically the radial flow type, these pumps operate with an impeller which helps pump water, the impeller operates by rotating really fast within its housing, this rotational movement of the impeller creates a low pressure area around the eye of the impeller which is the inlet where water passes through, this reduction in pressure creates a vacuum in the area and at this point, the surrounding air pressure would have been equal or less than the vapor pressure of the water causing the water to boil and vaporize.
When the water vapors produced gets past this area and starts moving towards the discharge of the pump which has higher air pressure, the water vapors rapidly condenses and changes back into liquid, this repeated action of changes in state caused by repeated pressure changes causes the water vapor to implode and create shock waves which leads to cyclic stress which would in time damage the pumps impeller or its housing.
- Waste water treatment using vacuum evaporators: this concept has found better application as an highly efficient energy saving way of treating wastewater, producing about 95% clean water when used, it’s a process whereby water is treated in a vacuum evaporator, with this method, waste water is transformed into 2 flows, one clean water and another which is the concentrated waste. Energy is saved using this method by vaporizing the water in a vacuum where low heat of about 35oC is needed to make the water vaporize due to the low pressure present in the vacuum evaporators.
This method is particularly useful when the wastewater contains a high salt content or toxic metals or microorganisms. The waste water is made to boil & vaporize by low heat, leaving the concentrated waste while the water vapor produced are channeled into a condenser where the vapors are subjected to a higher pressure to change them back into liquid water free of previous pollutants
Application of low pressure boiling in the food industry
In the food industry, vacuum evaporators are also used as a means to evaporate water from food so as to increase the shelf life and storage stability of the food produce. While heating the produce can normally achieve this goal, heating some food products would change the content of the products and this can lead to an overall decrease in the value of such product.
Food products such as fruits, evaporated milk, tomato paste, ketchup, sugar are produced in this manner George said and his mum replied, wow son this is all interesting but the next time I ask you to boil me some water just heat the damn thing up okay? Okay mum George said, I don’t know why you can’t just say “heat up some water for me” but okay.
The next morning George woke up and had some rumblings in the garage, he went to check what was happening and then he saw his mother trying to get the vacuum pump into her trunk, what are you doing mom, he asked, nothing she said curtly, you are taking the pump to work? he asked, so, what's it to you she replied, are you trying to show off what you learnt to your colleagues at work mom George asked, boy shut up and mind your damn business Mrs. Price said, George turned back and went inside his room with a grin on his face, he knew he'd just made his mom the star of the week at work. Wipe that grin off your face boy before I beat your ass six ways till Sunday Mrs. Price said and then got into her car and drove to work.



Water is indeed the weird material with a lot of interesting characteristics under different conditions.
It really is
Thanks for stopping by
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Boiling water without heat? Wow, amazing! It goes to prove how weird water can be.
You did an expository piece here
I'm glad you found it interesting...thank you
I remember one topic we did in high school: "the anomalous behavior is water" well, can we say water is not so normal afterall?
Its far from normal
Congratulations @thurllanie! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @thurllanie! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Congratulations @thurllanie! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board and compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Vote for us as a witness to get one more badge and upvotes from us with more power!